New Research Discovers Carriers of PKD1 and PKD2 Mutations (with Early Hypertension) Show Signs of Kidney Damage >5 Years Before Diagnosis
Three fast facts about our latest publication presented at ASHG and AHA
Kidney disease is common in the developed world and is a major cause of morbidity and mortality in the United States. In particular, autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic form of kidney disease, and affects approximately 1 in 500 to 1 in 1,000 individuals worldwide. The majority of genetic cases are due to mutations in the gene PKD1, with the second largest percentage attributed to PKD2.
Previously, patients with PKD1/2 variants were known to be at risk of kidney disease, but it was hard to specifically predict who would get sick. Also, while kidney damage is usually permanent, we can treat other health problems like heart issues that often come with it, as well as help mitigate how much kidney damage occurs.
With this publication, we sought to find out if we could use genomics to better understand when kidney damage starts so we can manage the disease better, lessen the damage, and help prolong life. To do this, we used data from the UK Biobank, as well as our health system partners (MUSC, HealthPartners, and Renown) to study how kidney disease develops and how genes might play a role so that we could find the best time to start treatments and understand how kidney function changes as the disease progresses, even before it’s officially diagnosed.
This resulted in an analysis presented at the American Society of Human Genetics (ASHG) and the American Heart Association (AHA), which discovered the following:
1. In the general population, PKD1/2 carriers develop hypertension a mean of 11 years before their kidney disease diagnosis, which is, on average, 10 years earlier than non-carriers. 30% of PKD1 carriers have hypertension by age 40, compared with 11% of PKD2 carriers and only 3% of non-carriers. Comparatively, it takes until age 50 for 30% of PKD2 carriers to develop hypertension and until age 64 for 30% of non-carriers to develop hypertension. After the onset of hypertension, the progression to kidney disease is rapid for PKD1 carriers and is associated with increased incidence of severe morbidity.
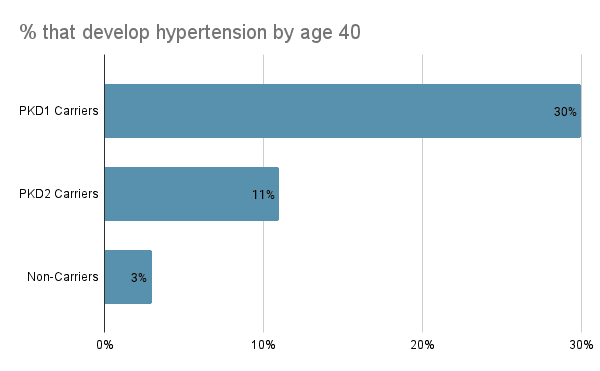
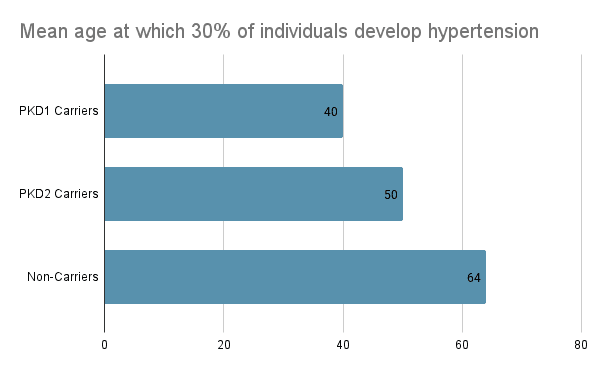
2. 85%+ of these PKD1/2 carriers with hypertension who aren’t diagnosed with the disease yet meet a key biomarker criteria for treatment an average of >5 years prior to their diagnoses. This suggests that many individuals are being diagnosed later than they should be. And a majority of these carriers have notable kidney damage well in advance of their diagnosis and already meet existing guidelines for treatment. If we could identify these people earlier, we might be able to greatly improve their outcomes.
3. As there are not currently population guidelines for screening for PKD1/2 variants, our analysis suggests that screening patients from the general population who develop hypertension before age 40 for PKD1/2 would identify that 1 out of 320 carry PKD1 or PKD2 mutations, and 75% of these individuals will go on to develop kidney disease. This would be a highly specific way to screen for the onset of kidney disease and is a prevalence that is similar to that seen for other genetic conditions like Lynch syndrome, and not far from the ACMG guidelines for genetic screening. Doing so would allow providers to stratify which patients they should consider monitoring regularly through regular serum creatinine tests because of their high risk for developing kidney disease in the future.
These findings are important because they show that autosomal dominant polycystic kidney disease could be anticipated and treated significantly earlier using a combination of targeted sequencing and routine monitoring.
For more information about this analysis and its results, please refer to our preprint:
Hypertension increases PPV for polycystic kidney disease in PKD1 and PKD2 variant carriers. It’s important to note that while this specific publication focuses on an early risk factor of kidney disease, a similar research approach can be taken and applied to disease states ranging from Cardio-Metabolic conditions like ATTR-CM to Neurodegenerative disorders including Alzheimer’s and Parkinson Disease. For another great example of this type of analysis, check out this paper. Please reach out to partners@helix.com to learn more or to discuss collaboration ideas with your organization.
Categories