The Case for Incorporating TTN Variant Testing into Routine Heart Disease Screening

With the rising popularity of wearable devices, more patients than ever are reporting self-diagnoses of atrial fibrillation to their doctors. Because early treatment of heart conditions can be critical in slowing their progression, identifying individuals in the early stages of heart failure may significantly improve their chances of delaying or preventing negative – or sometimes even fatal – health outcomes.
Our latest research on TTN variants in cardiology, performed in partnership with the Desert Research Institute, finds a direct genetic association between atrial fibrillation and cardiomyopathy, bringing new potential value to the implementation of population-level screening for this gene and incorporating genomics into routine management of heart disease.
Genetic testing in primary care
Amidst an explosion of real-world evidence (RWE)-based genomics research and genetic testing availability, there has been a growing need for more routine clinical implementation of genetics in primary care, as it has become more and more clear that average screening protocols do not adequately identify patients at-risk for diseases with known genetic associations.
For example, our 2018 study found that 90% of genetic risk carriers are not identified in typical clinical care, even though certain genetic carriers have an approximately 30 year acceleration of disease versus non-carriers.
What we knew before our analysis

In our most recent analysis, we dive into the TTN gene for a deeper understanding of its association with heart conditions in general populations.
A specific variant type within the TTN gene (cardiac truncating variants or TTNtvs) comprises the largest known genetic factor associated with cardiomyopathy. These variants are found nearly 20% of the time that sequencing is performed on patients diagnosed with this heart condition.
However, TTN variant testing has not had much clinical utility in the past because of its limited predictive ability to determine if a carrier will actually develop the disease.
What we found
In one of the largest longitudinal electronic health record (EHR) and exome analyses to date, we sequenced data from nearly 500,000 patients across two health systems. This analysis of ICD-based diagnosis patterns revealed TTNtv associations with both cardiomyopathy and atrial fibrillation, with evidence that these diagnoses together indicate a progressive form of heart disease.
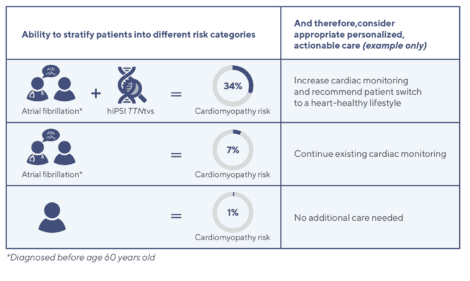
In fact, carriers with atrial fibrillation*, or 1 in 1000 people, had a 5-fold increased risk of developing cardiomyopathy versus non-carriers (34% vs. 7%), and a 47-fold increased risk versus the general population (34% vs. 1%).
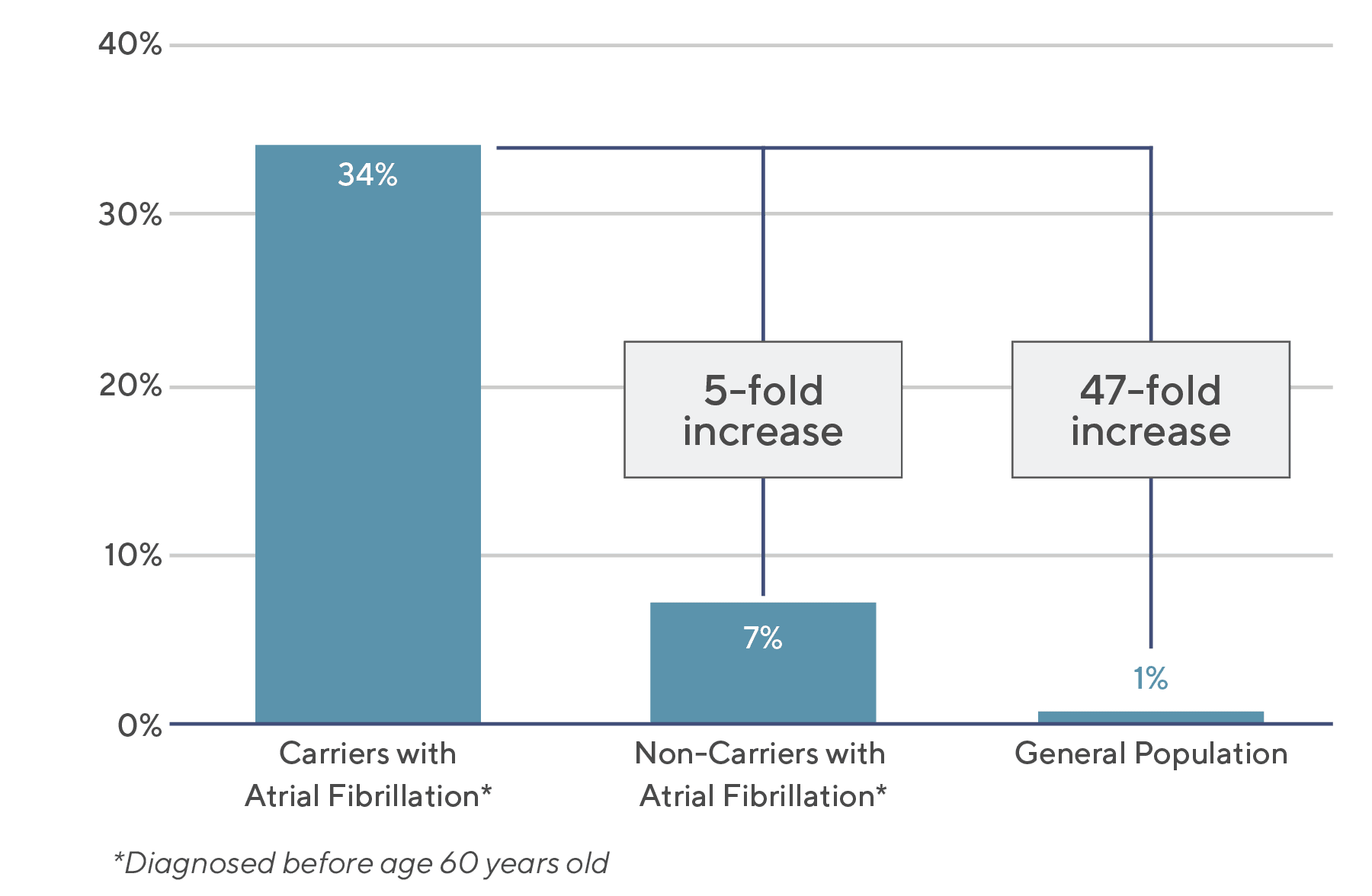
The study methodology and results are summarized in “TTN truncating variants in hiPSI exons show high penetrance for cardiomyopathy in carriers with atrial fibrillation”.
Implications for clinical care
Based on our findings, we conclude TTNtvs could serve as disease markers for elevated risk of cardiomyopathy, which could be evaluated in patients with atrial fibrillation or other early signs of heart conditions to inform more personalized screening and treatment – all of which could improve long-term patient outcomes. This is particularly useful as there are currently no screening recommendations for genetic cardiomyopathies outside of known family history.
These findings exemplify the value of data collected and analyzed through large-scale population genomics programs and its significance and relevance to improving clinical care. As a disease biomarker, TTN variant testing could also aid in characterizing the natural history of disease, as a target for new drug development and in recruitment for clinical trials, further reducing the disease burden of cardiomyopathy.
Advance your genetic sequencing capabilities with Helix
Are your service lines leveraging genomics to optimize and personalize care? With Helix exome sequencing and its end-to-end platform, health systems can streamline personalized medicine with a population genomics program that integrates with existing EHR workflows to improve health outcomes and accelerate research. Get in touch with our team to learn more about how a population genomics program could improve care, reduce costs and drive innovation at your organization.