Technical Briefing 6: JN.1
Executive summary
- JN.1, also known as BA.2.86.1.1, has been rising rapidly in recent weeks and is designated a variant of interest by the WHO.
- JN.1 accounted for either a similar or lower proportion of SARS-CoV-2 infections among hospitalized versus non-hospitalized patients in recent weeks, suggesting that the variant is not associated with more severe disease than cocirculating variants.
- Among all patients with medically attended SARS-CoV-2 infection, prior receipt of an updated fall 2023 vaccine was associated with a 67% lower odds of hospitalization compared to being unvaccinated. This association was similar when stratifying by variant, although the sample size for JN.1 infections is too small to say anything definitive yet.
Background
JN.1 (BA.2.86 + S:L455S) is currently rising rapidly in sequenced SARS-CoV-2 genomes worldwide. The WHO has designated it a variant of interest though it is currently evaluated as low-risk.
Given its rapid growth and the fact that JN.1 is a descendant of BA.2.86 – itself highly diverged from the XBB family of lineages – we investigate whether the new lineage is associated with any changes in severity or vaccine protection.
Data source
Helix has been collecting residual clinical samples from healthcare systems serving California, Minnesota, South Carolina, Wisconsin, and Washington under an IRB-approved research protocol. These samples are from individuals who were tested for a respiratory virus during diagnostic testing. In addition to the samples, Helix receives metadata associated with each sample, including collection date, individual’s age range, as well as the encounter type.
Unless otherwise indicated, this analysis included a total of 5,463 SARS-CoV-2 samples collected from patients between Jul 30, 2023 and Dec 16, 2023.
Rise of JN.1
During the two-week period spanning Dec 3-16, 2023, JN.1 infections accounted for 31.3% of all sequenced SARS-CoV-2 infections, compared with 10.7% during Nov 19-Dec 2, 4.0% during Nov 5-18, and <2% during each prior 2-week period (Figure 1).
Given that JN.1 accounted for an estimated 44.2% of SARS-CoV-2 infections nationally by Dec 23, 2023 (up from 21.3% during the prior two-week period, newly surpassing HV.1), we expect to see a similar subsequent ongoing rise of JN.1 in our data later in December 2023.
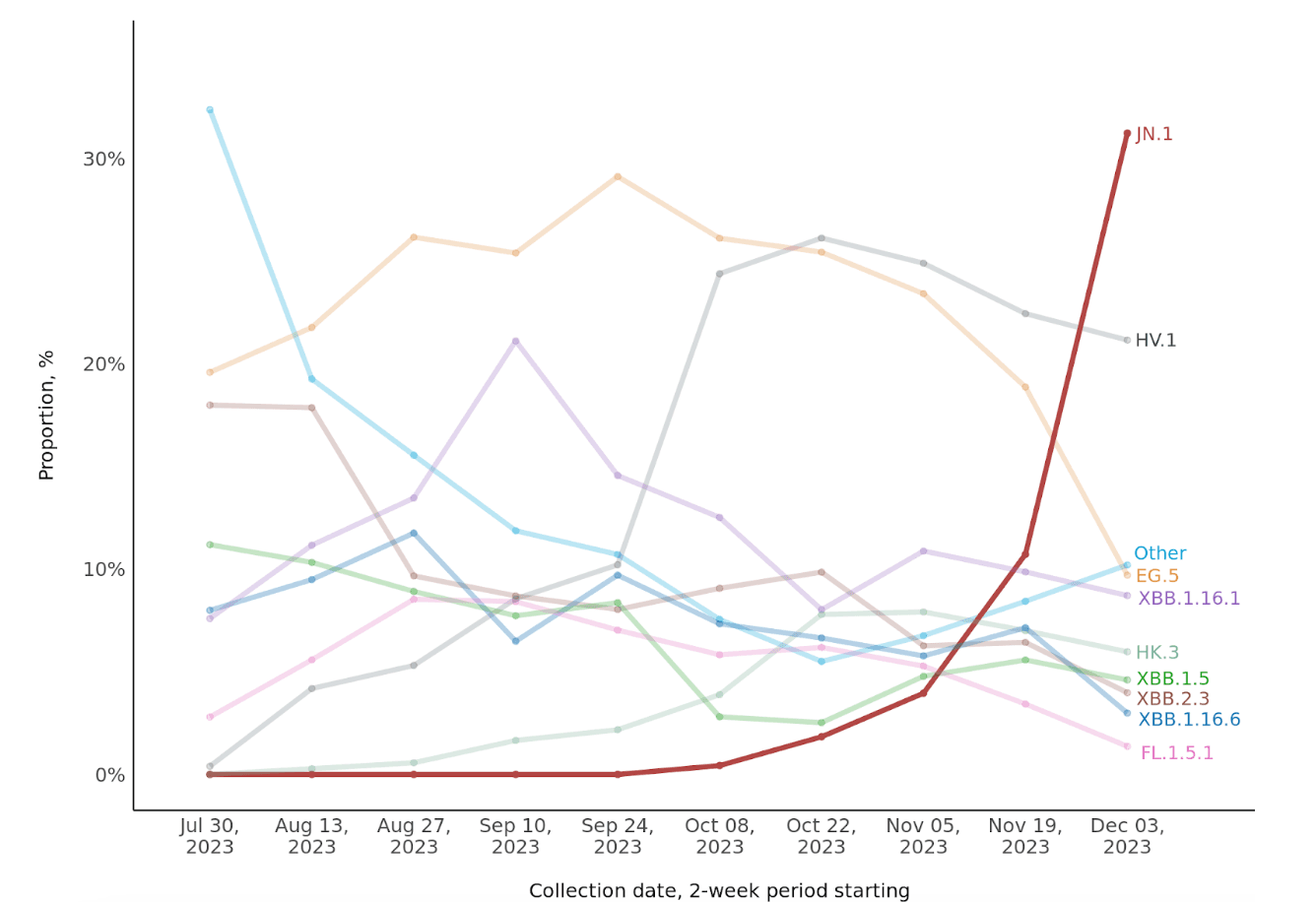
Figure 1: Biweekly proportions of JN.1 and other SARS-CoV-2 variants, July 30 to December 16, 2023. The total number of sequenced samples during the most recent two-week periods are 803 (Dec 3-16), 699 (Nov 19-Dec 2), and 606 (Nov 5-18). Except for HV.1 and HK.3, EG.5 sublineages are aggregated with EG.5. Sublineages of each other named lineage are aggregated with the respective lineage. JN.1 is also known as BA.2.86.1.1, EG.5 as XBB.1.9.2.5; FL.1.5.1 as XBB.1.9.1.1.5.1; HK.3 as XBB.1.9.2.5.1.1.3; and HV.1 as XBB.1.9.2.5.1.6.1.
Severity of JN.1 infections compared with cocirculating variants
The proportion of SARS-CoV-2 infections accounted for by JN.1 have been lower or similar among hospitalized versus non-hospitalized patients: 19.1% vs. 32.9% during Dec 3-16, 2023, respectively; 13.2% vs. 10.4% during Nov 19-Dec 2; and 2.5% vs. 4.2% during Nov 5-18 (Figure 2). This suggests that the JN.1 variant is not associated with more severe disease compared to other circulating variants. It will be important to continue to monitor this pattern as the total number of JN.1 infections rises.
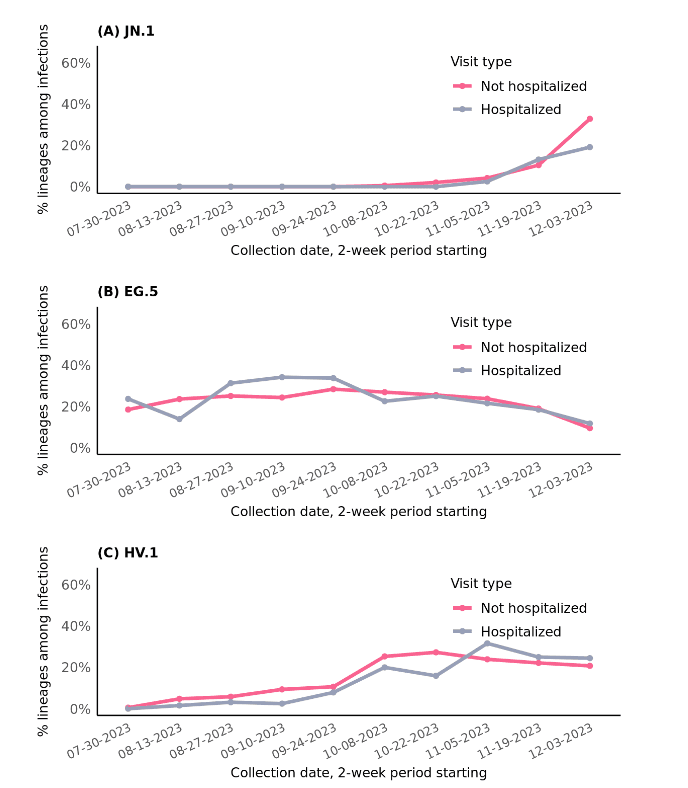
Figure 2: Relative proportions of JN.1, EG.5, and HV.1 variants among SARS-CoV-2 infections over time, stratified by visit type, July 30 to December 16, 2023.
SARS-CoV-2 vaccination and protection against hospitalization
Among all patients with medically attended SARS-CoV-2 infection, those who had received an updated fall 2023 SARS-CoV-2 vaccine at least seven days earlier were less likely to be hospitalized (10.1%) compared to patients who had never been vaccinated (14.1%) (Figure 3).
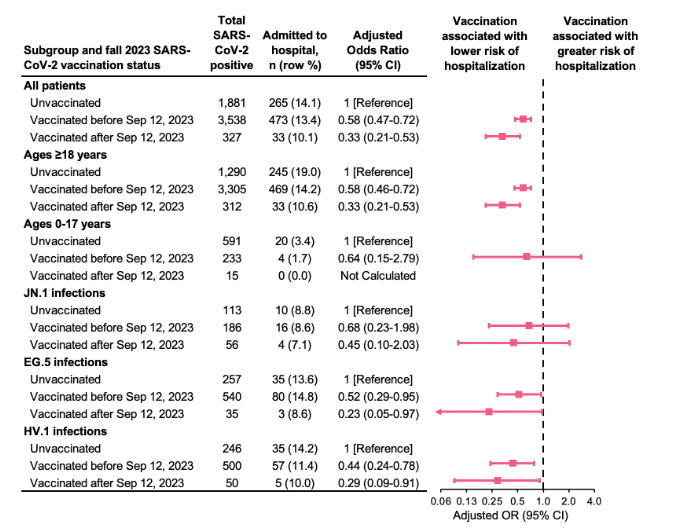
Figure 3: Association between prior SARS-CoV-2 vaccination and hospitalization overall and stratified by age group and SARS-CoV-2 variant, September 19 to December 16, 2023. Analyses were adjusted for health system, age, sex, race/ethnicity, and specimen collection date. Age and specimen collection date were smoothed using natural cubic splines. Receipt of an updated fall 2023 vaccine was defined as receipt of a SARS-CoV-2 vaccine on or after Sep 12, 2023. Patients vaccinated within 0-6 days before the specimen collection date were excluded.
After adjusting for demographic factors and calendar date, receipt of an updated fall 2023 vaccine was associated with a 67% lower odds of hospitalization compared to being unvaccinated (adjusted odds ratio [OR]=0.33; 95% confidence interval [CI]: 0.21-0.53). The association between vaccination and hospitalization was similarly protective, yet weaker, when comparing patients whose most recent vaccination was before fall 2023 to those who were unvaccinated (adjusted OR=0.58; 95% CI: 0.47-0.72).
This finding was largely driven by adult patients, as there were fewer pediatric patients in the sample and only a total of 24 hospitalizations among patients aged 0-17 years, which included zero hospitalizations among those who received the updated fall 2023 vaccine.
The magnitude and directionality of vaccination-hospitalization associations were similar among patients infected with JN.1, EG.5, and HV.1 variants, although precision was limited.
Caveats
Findings should be interpreted within the context of several limitations. First, documented diagnoses and symptomatology were not available for this analysis, precluding our ability to characterize patients’ diagnoses, signs, and symptoms related to acute respiratory illness. We were also unable to account for the presence of underlying medical conditions including immunocompromising conditions, which may have differed between vaccinated and unvaccinated individuals. Second, whether patients were hospitalized was defined using the location and visit type associated with the specimen collected for respiratory virus testing, and was not evaluated longitudinally over the course of one’s infection or illness. Third, misclassification of vaccination status is possible if vaccination records were incomplete. Fourth, when stratifying by variant among recent infections, some sample sizes were smaller than optimal at the time of the current analysis (e.g., JN.1 infections).
Prepared by Shishi Luo and Matt Levy.
© Helix, Inc. 2023. All rights reserved.
Helix and the Helix logo are registered trademarks of Helix, Inc.
Categories