Technical Briefing 2: COVID-19 Rebounds
Executive summary
- In pharmacy-based PCR testing, rebounds account for 2.2% percent of infections in high-frequency testers.
- As a fraction of infections in all testers, rebounds comprise only 0.16%, suggesting that most rebound cases go unnoticed.
- We do not find evidence that rebounds increased in frequency after Paxlovid became available.
Background
Rebounds have been described as a resumption of symptoms and a positive test, following a negative test. Early cases of rebound have involved patients on Paxlovid and cases have also been mentioned in the Emergency Use Authorization (EUA) for Paxlovid. Notably, both the President of the United States, Joe Biden, and his Chief Medical Advisor, Anthony Fauci, experienced rebounds after taking Paxlovid. The mechanism underlying viral rebound is still not well understood, although evidence so far suggests it is caused by a resurgence of the same infecting virus, rather than by a mutated variant that is able to escape the immune response. Here we use commercial PCR test data from retail pharmacy testing to quantify the frequency of rebounds and investigate whether rebounds increased with Paxlovid availability.
Data source
This analysis uses 1,244,791 PCR test results from October 10, 2020 to August 10, 2022, representing 442,204 patients who have tested with Helix through a retail pharmacy in the United States and received at least one positive test result. Of these 1,244,791 tests, 558,340 are positive. These positive tests represent 449,759 unique infections, defined as positive tests more than 28 days apart. The data set analyzed does not contain prescription information, symptom information, or other patient demographic information (such as age).
Rebound frequency
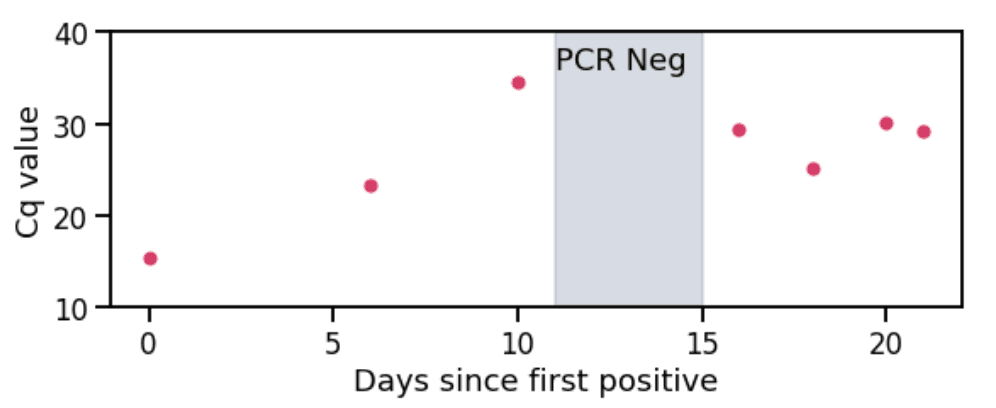
Figure 1. Cq values (cycle threshold quantity) over time from a rebound case. Shaded region indicates period where the patient received a negative test result. Low Cq values indicate high viral load and high Cq values indicate low viral load.
Rebounds are defined here as a positive-negative-positive test result pattern within a period of 28 days (see Figure 1 for an example). There are limitations to using this as a proxy for rebounds and these are discussed in the ‘Caveats’ section.
We calculate the fraction of rebounds in two groups: all testers and high-frequency testers. High-frequency testers (21,362 or 0.5% of testers in our dataset) are defined as individuals who have tested at least three times and for which the median time between tests is at most a week. These are likely to be individuals who are obliged to test regularly, either for work or school purposes.
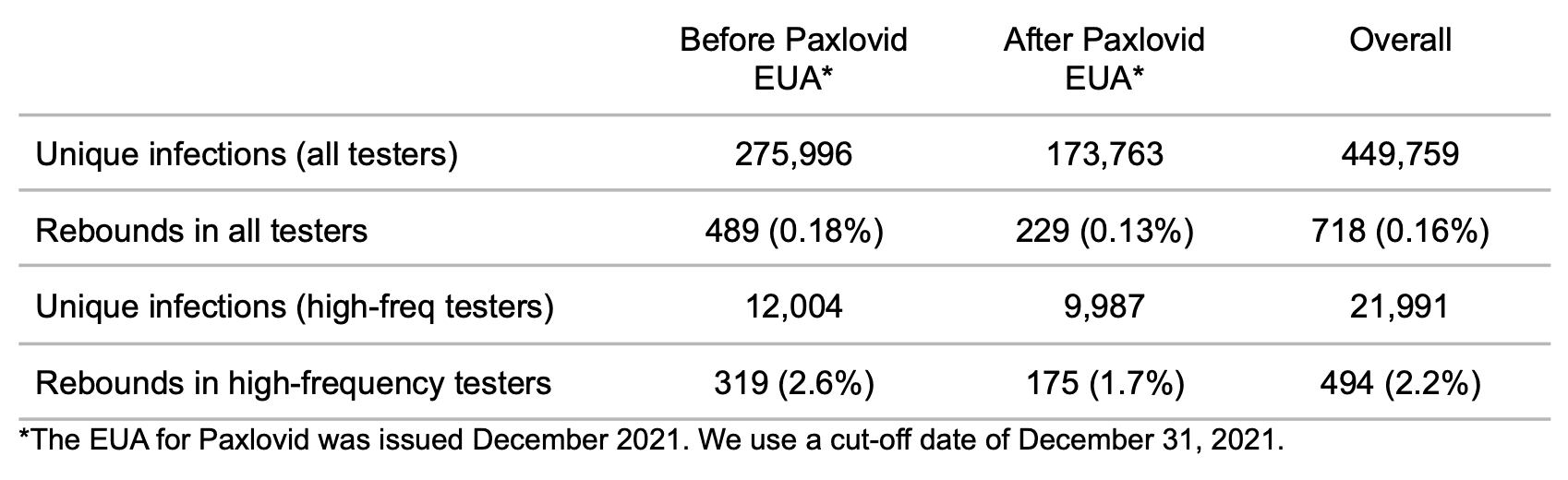
Table 1. Frequency of rebounds.
Most rebounds are not detected
A naive calculation gives a 0.16% rate of rebound occurrence as a fraction of infections in all testers. When we restrict our calculation to infections in high-frequency testers, where rebounds are more likely to be caught, the rate is much higher: 2.2%. This value is closer to the 3.53-5.40%, 1.73-2.32%, and 1-2% ranges that have been reported in other studies. This suggests that most rebound cases are not being detected in PCR testing, probably because individuals who have tested negative after an infection will typically not test again, believing their infection to be over. On the other hand, individuals who test regularly regardless of their infection status will pick up on viral rebounds. This may explain why there are so many high profile cases of Paxlovid rebound; due to the nature of their work, high profile individuals are more likely to test regularly.
Rebounds have not increased in frequency since Paxlovid became available
Within the high-frequency testing group, the frequency of rebounds decreased from 2.6% to 1.7% after Paxlovid received authorization. Assuming that our sample includes Paxlovid-treated infections at the same proportion as the general population, this suggests that Paxlovid treatment does not increase the chance of rebound. This result is supported by a study which found that rebounds occurred at a similar frequency in patients taking a non-Paxlovid antiviral.
Caveats
This analysis does not have patient-level information about prescriptions. Instead we infer, based on the date of the Paxlovid EUA, whether increased use of Paxlovid would cause an increase in rebounds in the aggregate.
Rebounds may not always manifest as a sequence of positive-negative-positive results in PCR tests. It is possible that PCR tests could be positive for the entire period of a rebound (as in this case study). An alternative way to detect rebounds in PCR data is thus to apply signal processing on the Cq trajectories of all infections. Unfortunately, we do not have enough samples with sufficient granularity of Cq values over time for this analysis to be meaningful. We instead use a sequence of positive-negative-positive, which requires only three tests during an infection.
Limitations of using the positive-negative-positive pattern include: (1) the intervening negative may be a false negative caused by low viral load, (2) the second positive may be a reinfection instead of a rebound. Regarding the first point, the left plot in Figure 2 indicates that a large fraction of the rebound positives indeed have high Cq (i.e. low viral load). This could mean that the intervening negative was false. It could also mean that a rebound occurred, but that the viral load during the rebound was low. Regarding the second point, the right plot in Figure 2 shows that the majority of the rebounds occur within a period of two weeks. The ones occurring beyond two weeks could arguably be characterized as reinfections.
These caveats highlight the difficulty of defining a rebound without clinical information and without a hypothesis for its etiology.
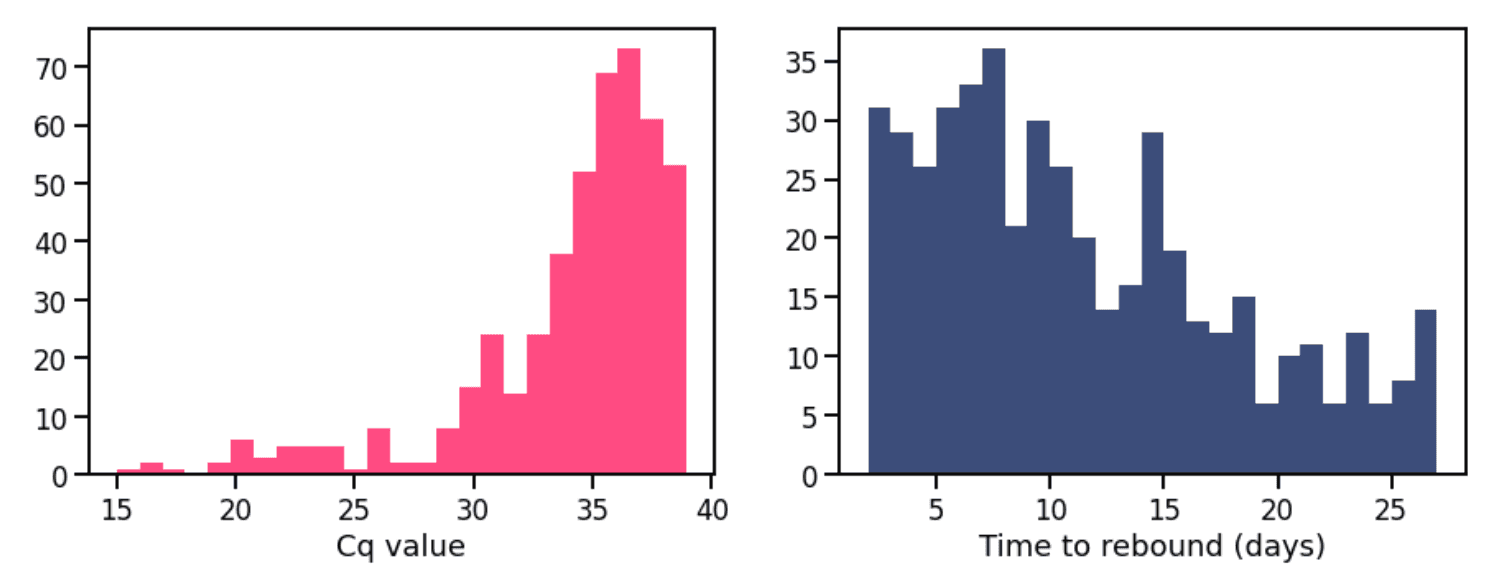
Figure 2. (Left) Frequency of Cq values for first positive test in rebound. (Right) Frequency for time between last positive test in initial infection and first positive test in rebound.
Prepared by Shishi Luo and JT McCrone. Special thanks to Simon White for pulling the data.
Disclaimer: Any opinions, findings, and conclusions expressed in this technical briefing are those of the authors and do not necessarily reflect the views of Pfizer, the manufacturer of Paxlovid.
Categories