Helix survey data contributes to a new COVID-19 study

In a recent study, researchers from an international consortium used genetic data alongside survey responses to gain insight into how our DNA may affect our vulnerability to the SARS-CoV-2 virus (the virus responsible for the COVID-19 pandemic).
For this unique study, the researchers combined data from four different populations—including Helix’s research community—and estimated how many people were likely to have been infected by SARS-CoV-2 based on their reported symptoms. The team took it a step further by analyzing DNA from those who were estimated to have been infected with SARS-CoV-2 and compared it to DNA from those who had likely not been infected.
Out of this analysis, two genetic factors stood out as possibly having an influence on vulnerability to COVID-19. Research into these two factors will be ongoing, and many more independent studies will be needed to confirm their findings. Nonetheless, the results reported in this preprint are an exciting development that adds to the growing body of research on COVID-19.
Background
Humans have always had a close relationship with viruses, albeit an antagonistic one at times. Viruses are part of the complex ecosystem that makes up the human gut microbiome, and portions of viral DNA can even be found within the human genome—relics of ancient infections that no longer produce viruses but have nonetheless persisted through generations of human history.1,2
Occasionally, we encounter viruses that are truly dangerous. The SARS-CoV-2 virus is a prominent example—this virus is responsible for the ongoing COVID-19 pandemic. And while we know a great deal about viruses, this particular one has several mysteries that limit our ability to combat it.
One such mystery has to do with the wide variety of symptoms that people seem to experience after being infected. Multiple studies have reported that at any given time, 50-90% of infected individuals have no overt symptoms whereas others become critically ill3-8.
So why doesn’t everyone respond in the same way?
In looking back at our history with viruses and past pandemics, scientists have found various factors that may shed light on our current situation, including genetics. Research into the human immunodeficiency virus (HIV) revealed that some people were less vulnerable to infection owing to a heritable change in their DNA that affected the ability of the virus to invade (and persist) in the human body.9-11 In this example, we see that people’s DNA can differ in small ways and these differences can sometimes affect their vulnerability to viruses.
The same may be true for SARS-CoV-2.
Using symptom-based case predictions to identify host genetic factors that contribute to COVID-19 susceptibility
Researchers around the world have joined together in consortia to perform large-scale genetic studies. The sharing of data for these studies is critical because the more data you can get, the more likely you are to discover subtle but important patterns.
For this study, researchers decided to use four large research populations, one of which was the Helix research community. With data from surveys in which respondents reported any symptoms they may have experienced, researchers were able to “predict” who was likely to have had COVID-19 in the past several months. This prediction was based on previous studies focused on which symptoms were more frequent among people who had tested positive.
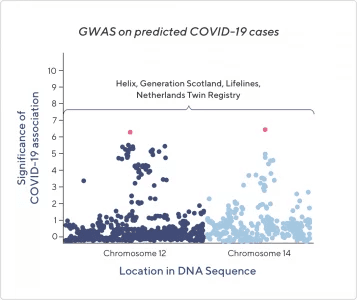
The above image is a stylized representation of data from this study’s GWAS, which stands for Genome-Wide Association Study—a common technique in genetics research. Each dot represents a specific genetic factor that differs between people. The y-axis represents the significance of the factor’s association with COVID-19 symptoms—the higher the number, the more likely it is that this factor is related to symptoms of COVID-19. The two pink dots represent the two factors most associated with COVID-19 symptoms. The x-axis just represents the physical location along each chromosome where the genetic factor can be found.
In comparing DNA from people who tested positive or were predicted to have had COVID-19 against those who tested negative or were predicted to have not had COVID-19, the researchers were able to find two genetic factors that appear to be associated with having COVID-19 (more specifically, with having symptoms that would qualify you as a “predicted positive” case).
It’s not clear how these genetic factors may influence a person’s vulnerability to COVID-19, but it may relate to how cells respond to viral infection. As with most findings in research, more research will be needed to make sense of this study’s findings.
Nonetheless, this study managed to take several important steps forward. Identifying these two factors will help direct future research efforts. Additionally, the use of predicted positive cases in this study demonstrates that it can be a useful tactic when limited testing is available.
Disclaimer
While this research method demonstrated a statistically significant ability to predict who may have had COVID-19, based on the large-scale analysis of certain genetic factors and self-reported symptoms, the research is still very new and may change overtime. Although the research may contribute to the development of diagnostic criteria and have other future applications in the clinical setting, researchers are not currently able to predict disease susceptibility with sufficient certainty to conclusively diagnose COVID-19 at the individual level. Additionally, in order to preserve the privacy of research participants, all of the data used for the study was de-identified. This means that researchers are unable to identify individuals in our cohort who were predicted to have had COVID-19 based on their symptoms. If you are concerned that you may have COVID-19, please see a healthcare provider.
Research continues
The study highlighted here is one of many projects that Helix scientists are working on. Some of the topics we’re focused on include how genetics may affect long-term symptoms of COVID-19, and what role genetic variations may play in the immune response to COVID-19. We’re proud to have an engaged and enthusiastic research community who is continuing to band together and work towards a better understanding of this pandemic. Everyone–from those filling out our survey to the researchers writing the papers—are in this together, and for that, we’re extremely grateful!
1. Markovitz, David M. “”Reverse genomics” and human endogenous retroviruses.” Transactions of the American Clinical and Climatological Association vol. 125 (2014): 57-62; discussion 62-3.
2. Mukhopadhya, Indrani et al. “The gut virome: the ‘missing link’ between gut bacteria and host immunity?.” Therapeutic advances in gastroenterology vol. 12 1756284819836620. 25 Mar. 2019, doi:10.1177/1756284819836620
3. Oran, Daniel P., and Eric J. Topol. 2020. “Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review.” Annals of Internal Medicine, June. https://doi.org/10.7326/M20-3012.
4. Ferretti, Luca, Chris Wymant, Michelle Kendall, Lele Zhao, Anel Nurtay, Lucie Abeler-Dörner, Michael Parker, David Bonsall, and Christophe Fraser. 2020. “Quantifying SARS-CoV-2 Transmission Suggests Epidemic Control with Digital Contact Tracing.” Science 368 (6491). https://doi.org/10.1126/science.abb6936.
5. He, Xi, Eric H. Y. Lau, Peng Wu, Xilong Deng, Jian Wang, Xinxin Hao, Yiu Chung Lau, et al. 2020. “Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19.” Nature Medicine 26 (5): 672–75.
6. Li, Ruiyun, Sen Pei, Bin Chen, Yimeng Song, Tao Zhang, Wan Yang, and Jeffrey Shaman. 2020. “Substantial Undocumented Infection Facilitates the Rapid Dissemination of Novel Coronavirus (SARS-CoV-2).” Science 368 (6490): 489–93.
7. Arons, Melissa M., Kelly M. Hatfield, Sujan C. Reddy, Anne Kimball, Allison James, Jesica R. Jacobs, Joanne Taylor, et al. 2020. “Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility.” The New England Journal of Medicine 382 (22): 2081–90.
8. Lauer, Stephen A., Kyra H. Grantz, Qifang Bi, Forrest K. Jones, Qulu Zheng, Hannah R. Meredith, Andrew S. Azman, Nicholas G. Reich, and Justin Lessler. 2020. “The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application.” Annals of Internal Medicine 172 (9): 577–82.
9. Liu, R. et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86, 367–377 (1996).
10. Samson, M. et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996).
11. Dean, M. et al. Genetic Restriction of HIV-1 Infection and Progression to AIDS by a Deletion Allele of the CKR5 Structural Gene. Science 273, 1856–1862 (1996).
Categories