The versatile and flexible Exome+ assay

Get the performance of a targeted panel, the breadth of a microarray, and the completeness of an exome – all from one assay
Since the founding of Helix in 2015, we’ve been continually optimizing our proprietary Exome+TM assay. Through custom development work and proprietary bioinformatics solutions, we’ve created an assay that meets the needs of both clinicians and researchers in contrast to off-the-shelf assays that are typically optimized for one or the other. Read on to learn how we’ve eliminated the compromises clinicians and researchers typically have to make on cost, quality, and breadth when choosing an assay.
What is the Exome+ assay?
The Exome+ assay is a panel-grade clinical exome enhanced by ~300,000 informative non-coding regions (and tens of millions of imputed variants). The design of this assay ensures that each base within clinically relevant regions are assigned high-confidence genotypes. We designate the Exome+ assay as panel-grade based on its ≥ 99.5% call rate across ~ 600 genes that are considered important in the areas of hereditary cancer, cardiovascular disease, pharmacogenomics, and carrier screening (Table 1). Small variant types detected and reported in these genes include SNPs, indels, multinucleotide variants (MNVs), substitutions, and complex variants. Large variants include both intragenic copy number variants (CNVs) and multigenic CNVs.
Capturing the best of both worlds: Targeted panel + whole exome
Panels are often used in clinical settings because they’re low cost and provide high quality coverage of specific, pre-defined genomic regions of interest. While useful in certain settings, such assays are limited by their narrow focus. For example, if it becomes necessary for either research or clinical purposes to examine parts of a patient’s genome that fall outside of the panel, another test would need to be ordered. This is both costly and time consuming.
Standard off-the-shelf whole exome assays offer much broader coverage but are known to be incomplete and to suffer from gaps in coverage. Because of this, exome sequencing is generally inappropriate for clinical applications that require panel-grade testing—tests that can match or exceed the accuracy of panels.
Helix’s Exome+TM assay has been carefully optimized to provide the benefits of targeted panels and the completeness of whole exome sequencing.
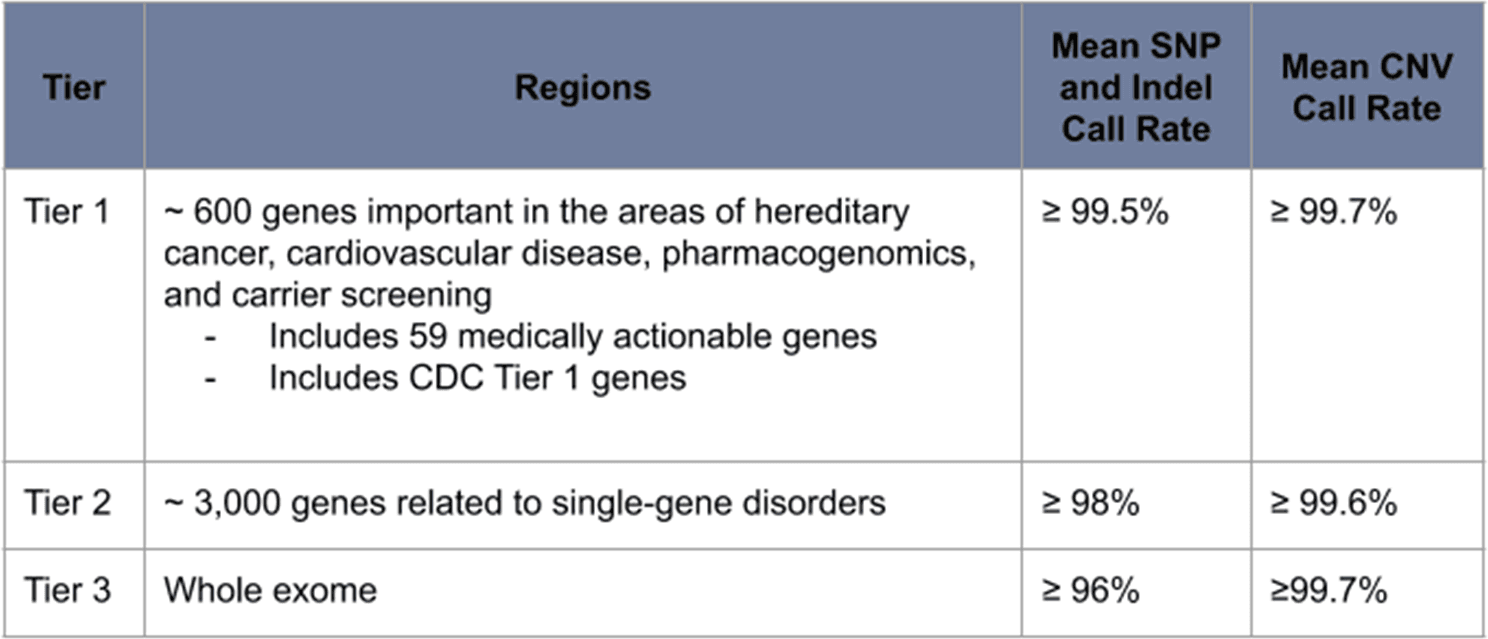
Table 1: Call rates are optimized for clinically relevant portions of the exome.
Quality and affordability through uniform coverage
Helix’s Exome+ assay maintains a consistently high call rate due in large part to our focus on optimizing uniformity of coverage across all coding regions. The result is a relatively even distribution of reads across the exome, ensuring consistent base-to-base call rates (see Box for more on call rates). Importantly, the even coverage provided by the Exome+ assay enables it to remain low-cost: many assays attempt to overcome their deficiencies by over-sequencing various parts of the exome which can be an expensive endeavor.
Box 1: Coverage versus Call Rate:
We work hard with every iteration of the Exome+ assay to optimize for uniform read distribution across regions that are important for specific types of genetic testing. For these genes, we provide high-confidence calls at a rate of ≥99.5%, with base-by-base coverage at >20x coverage.
It’s common to describe coverage across regions of interest; for example, “mean coverage of 100x”. But coverage only tells part of the story. What is important to any clinician or scientist is what percentage of the region receives trustable results, also known as the call rate.
Using average coverage to infer call rate is a faulty practice because an assay’s coverage can vary greatly within a genome, exome, or panel. For example, regions may have reduced coverage due to high or low GC content; or coverage may be low as a result of other similar sequences that reduce the mapping quality of the reads. This means a test with an average of 100x coverage may still have large regions with no coverage at all, though the assay’s overall average remains high. High coverage may, in some cases, translate to higher prices for a less efficient sequencing assay.
Gaps in coverage are never obvious in coverage discussions; they’re revealed only when discussing base-by-base call rates. Learn more about our call rates on medically relevant regions in our clinical white paper.
Microarrays and lcWGS: Capturing the benefits and addressing the shortcomings
In the past, many large scale genomic studies relied on microarrays as a compromise to get genetic data from a large number of individuals without breaking the bank. As the cost of sequencing has gone down, however, more researchers are turning to low-coverage whole genome sequencing (lcWGS)—typically defined as 0.1 – 3x mean coverage—to achieve similar results. lcWGS and microarrays are powerful tools for GWAS and evaluating polygenic risks scores, but cannot be applied to panel testing due to the lower accuracy of individual variant calls and the incompleteness of gene coverage.
With the design of the Exome+ assay, we’ve harnessed the utility of lcWGS and microarrays by directly covering 300,000 non-coding regions which enables genome-wide imputation (and is also why we can return ancestry results). Imputation accuracy of the Exome+ is comparable to 0.5x lcWGS.
The benefit of a panel-grade clinical exome: It’s future proofed.
There are several advantages to running panels in the presence of a whole exome, including flexible test design, flexible re-testing, and increased functionality.
Flexible Test Design
With the Exome+ assay, ~20,000 genes are sequenced for every sample. This means that when designing a panel off of the Exome+ assay, every gene is a candidate for inclusion in the panel. Panels can be customized and, as relevant, quickly expanded. We have ensured the highest call rates across those genes that are most likely to belong to a panel; however, the Exome+ assay delivers high call rates for all genes beyond this core set (Table 1).
For example, in cardiovascular disease, it’s often preferred to use a test that includes a large number of genes; and, it’s common for these tests to evolve over time to include more genes. Delivering panels off of the Exome+ assay supports the flexibility needed to ensure that a cardiovascular test is both comprehensive and current.
Flexible Retesting
The Exome+ assay supports many panels off of a single assay. This means that once an individual has been sequenced and tested for one indication, the data is available and can be retested for additional indications as needed (Figure 1).
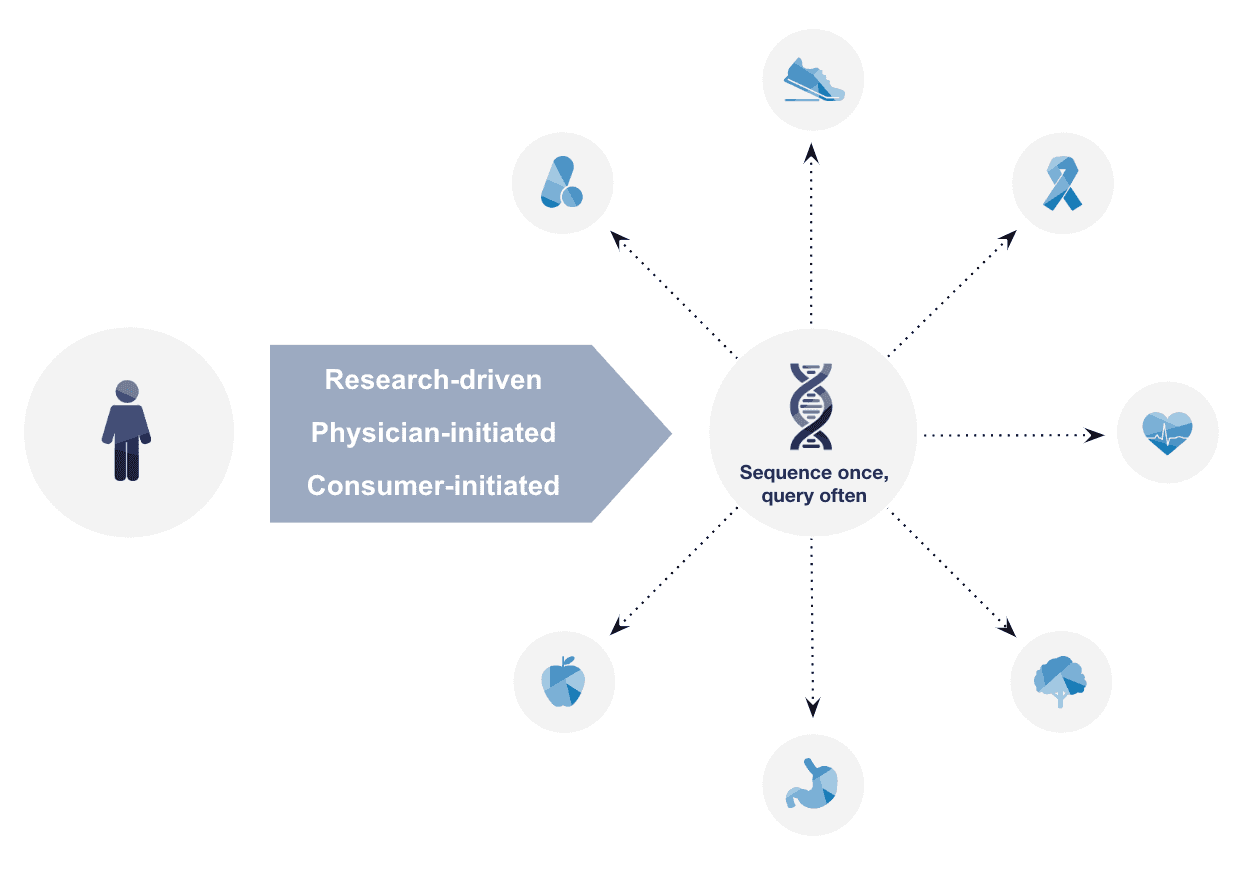
Figure 1: Data generated using the Exome+ assay can be used repeatedly over time for various purposes, including disease specific screening, pharmacogenomics, and research.
Increased functionality
In addition to achieving parity with targeted panels and exomes, the Exome+ assay enables superior pharmacogenomics—the use of this data to predict drug response based on variants across genes involved in drug metabolism. Unlike panel testing, which often focuses on individual variants within a gene, pharmacogenomic testing is based on the combination of variants present throughout a gene’s sequence (referred to as the gene’s star alleles). Helix’s Exome+ assay includes proprietary bioinformatics solutions which enable reporting on all known star alleles, rather than just what is expected. By way of example, the Helix PGx bioinformatic pipeline reports out 106 star alleles along with whole gene copy number variation for CYP2D6. Typical commercial assays only report on the most common star alleles, leading to mischaracterization of CYP2D6 in over 15% of samples. In avoiding this mischaracterization, the Exome+ assay provides a superior, comprehensive pharmacogenomics solution.
One assay, many uses
The Exome+ assay covers SNPs, indels, CNVs, and ~300,000 non-coding portions of the genome (as well as tens of millions of imputed SNPs). Whether you’re looking to discover novel biomarkers or return clinical-grade results to patients, the Exome+ assay has you covered. Learn more from our white papers (Performance white paper, clinical applications) or get in touch at partners@helix.com
Categories