Saliva-based COVID-19 testing is less sensitive than nasopharyngeal swabs in the community setting
Helix researchers, along with collaborators from Renown Health and UC San Diego, found that, relative to nasopharyngeal swabs, the sensitivity of saliva was approximately 30% lower in a community-based diagnostic cohort and 50% lower in a convalescent cohort
Accurate and scalable testing for COVID-19 is a critical part of any strategy to enable communities to return to normal activities. Today, the vast majority of COVID-19 tests are conducted by collecting samples with nasopharyngeal swabs (NPS). This approach to sample collection is challenging to scale broadly because of the need to involve a healthcare provider, higher risk of viral transmission to healthcare workers (requiring PPE), and significant patient discomfort. There are also shortages of swabs and viral transport media and many health systems are struggling to get enough supplies to test at the levels they need to.
Self-administered saliva collection has the potential to overcome these limitations and recent EUA approvals of saliva-based collection (with data generated in a symptomatic, hospital setting) have sparked significant interest in this approach.1,2 Because we have years of experience collecting and processing saliva samples at large scale, we were eager to put that knowledge and infrastructure to use to help meet the need for accurate and scalable testing. Along with researchers from Renown Health and UC San Diego, we set out to investigate whether saliva-based testing was a suitable alternative to NPS in the community setting. Unfortunately, our findings, available as a pre-print on medRxiv, show that the reduced sensitivity of saliva relative to NPS makes it unlikely to be the solution for large-scale community testing.
James Lu, M.D., Ph.D., co-founder and Chief Scientific Officer at Helix and senior author of the study, said:
Based on the lower sensitivity of saliva relative to NPS, our view is that saliva will not be a viable sample type for either community testing or for determining back-to-work status
Our approach and findings
The focus of this research was to determine whether saliva-based testing for COVID-19 was sensitive enough to detect COVID-19 in the community setting, where patients are likely less symptomatic and thus where viral loads are likely lower. We focused on two specific use cases that are critical to loosening social distancing guidelines:
- Diagnosing new COVID-19 infections in the community setting.
- Determining if someone is no longer infectious after contracting COVID-19 and thus safe to come into contact with uninfected individuals, such as in a “back to work” scenario.
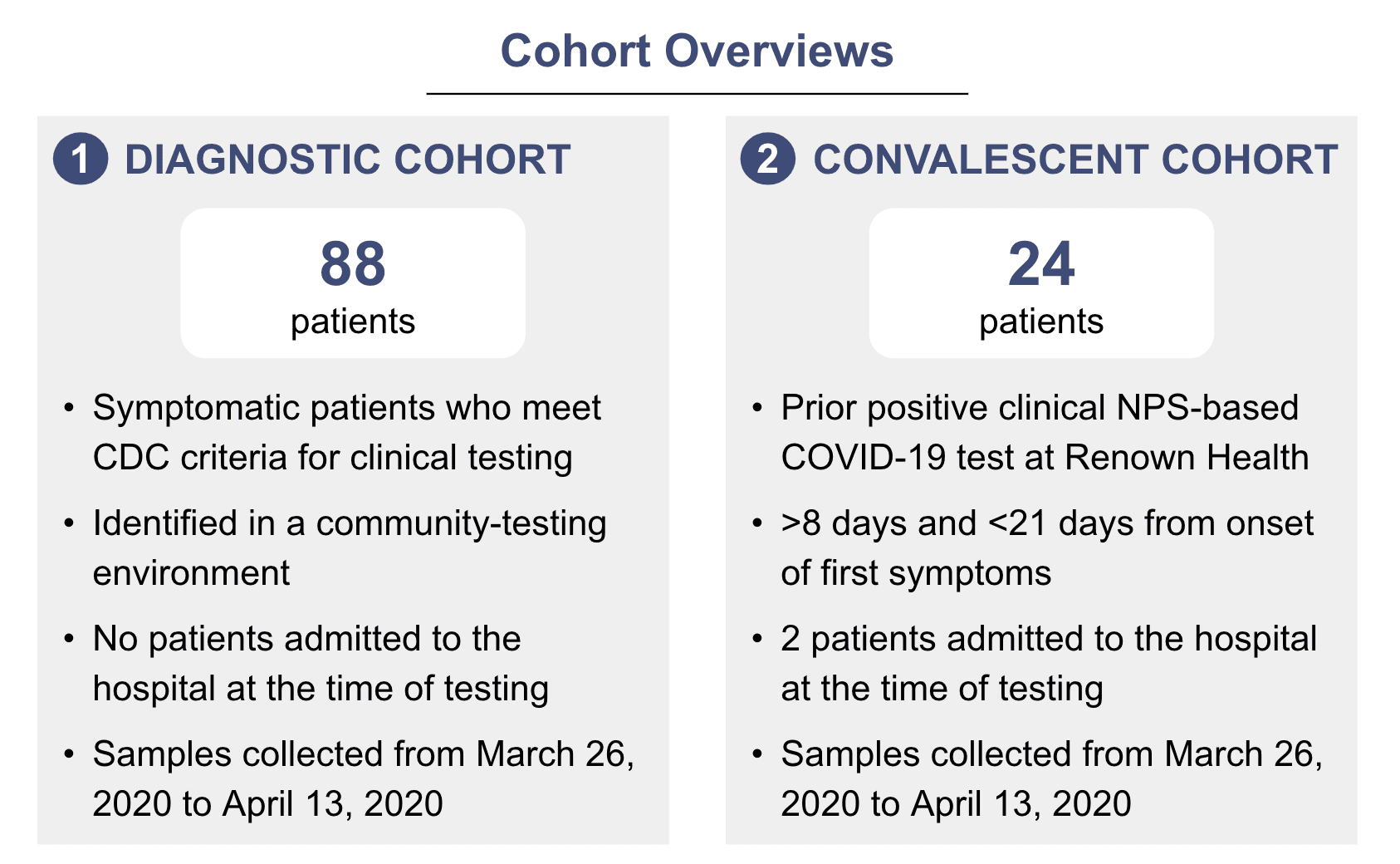
To evaluate the utility of saliva-based testing for these use cases, we collected paired NPS and saliva samples from two cohorts of patients – a “diagnostic cohort”, to evaluate the utility of saliva in detecting new infections, and a “convalescent cohort,” to determine if saliva is sensitive enough to confirm if someone is no longer infectious.
The Diagnostic Cohort
We enrolled and consented a total of 88 individuals who presented and qualified for testing in the Diagnostic Cohort to evaluate whether self-collected saliva could alleviate some of these challenges. NPS and saliva samples were collected simultaneously from each individual.
NPS samples were sent to the Nevada State Health Lab, which used the CDC RT-qPCR assay for diagnostic testing. Saliva samples were sent to Helix, where RNA was extracted and evaluated using the PrimerDesign COVID-19 assay performed at Helix and the TaqPath Multiplex RT-PCR COVID-19 assay performed at UC San Diego.
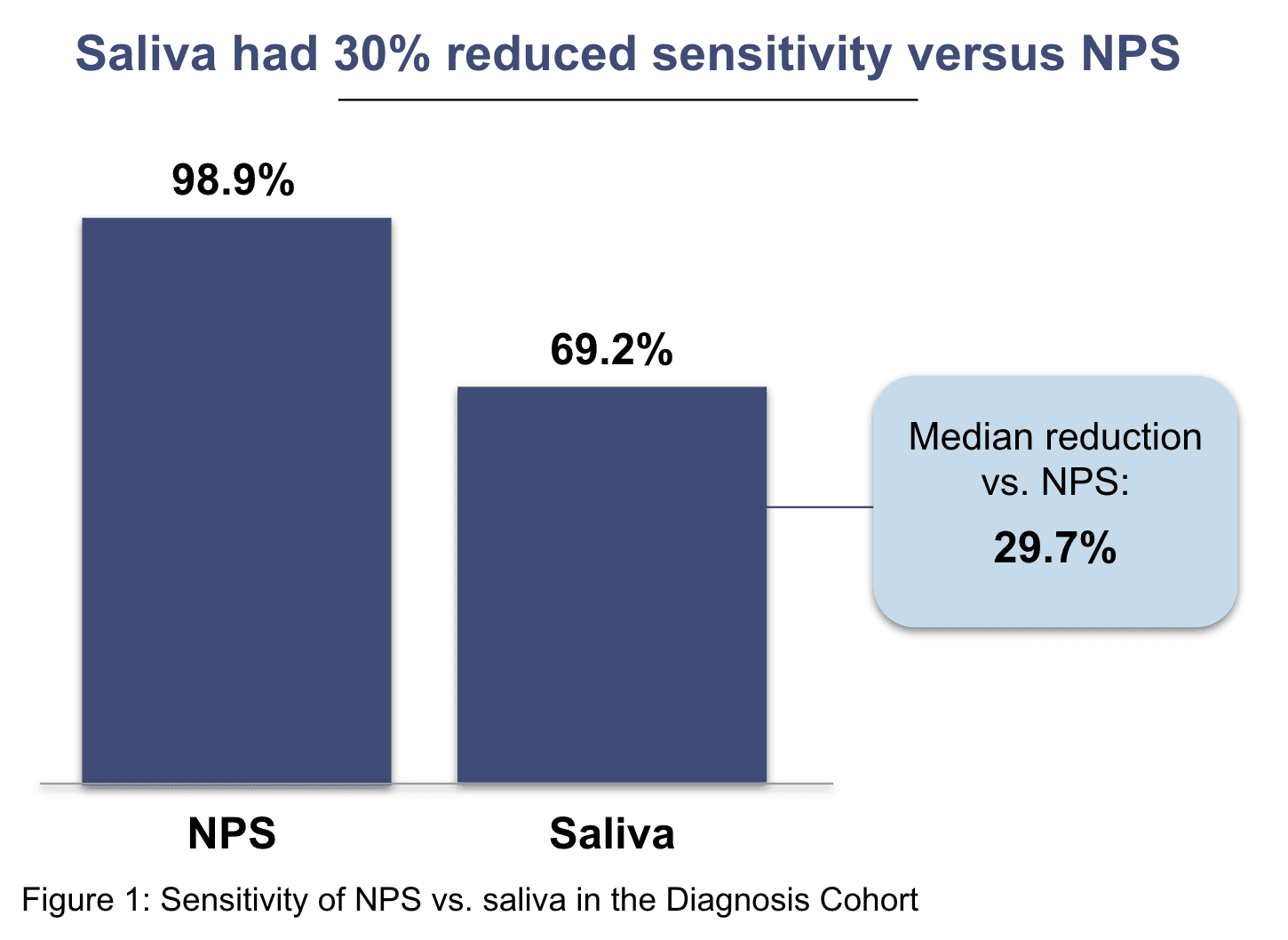
In this cohort, we found that saliva has a lower sensitivity (~29.7% reduction) relative to NPS.3 This implies that if 100 mildly symptomatic COVID-10 positive patients were tested with saliva, ~30 would receive false negative results – meaning they would be told they do not have COVID-19, when in fact they do.
The Convalescent Cohort
In addition to detecting new COVID-19 infections, we also need to be able to test people after they’ve recovered from COVID-19 symptoms to determine whether they’re still infectious. For some patients, this might need to be done several times – for instance, the CDC recommends that healthcare workers have two negative tests in a row before returning to work.4 To assess whether saliva would be feasible for this use case, we evaluated 24 confirmed COVID-19 patients, most of whom were 8 days or more from the onset of first symptoms with paired NPS and saliva samples. RNA was extracted from the NPS and saliva samples and analyzed using the PrimerDesign and TaqPath RT-PCR assays at Helix and UC San Diego laboratories.
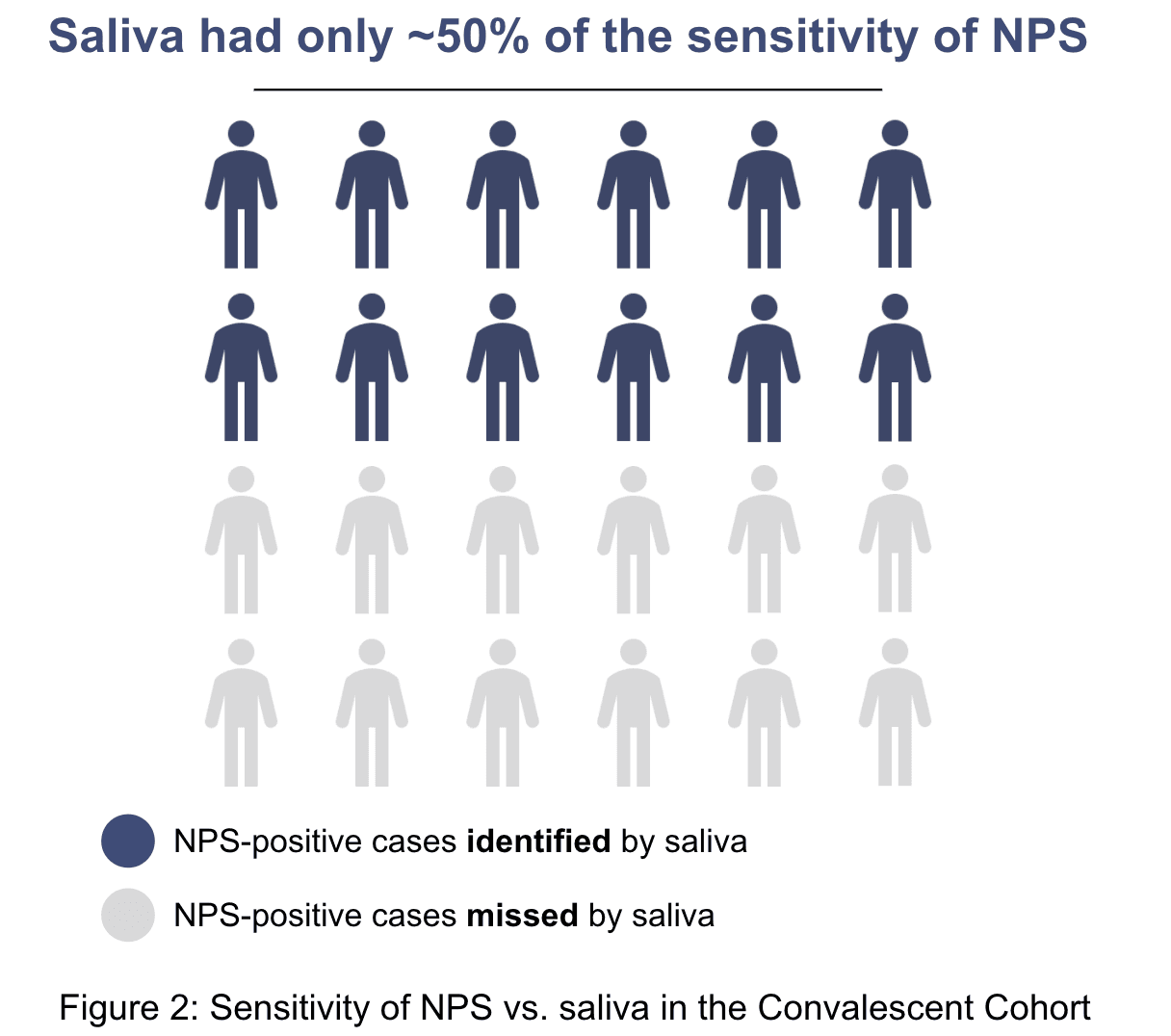
Overall, the sensitivity using the NPS samples was higher than for the saliva samples. We found that saliva missed approximately 50% of individuals who were positive on nasopharyngeal swabs. This implies that if 100 people were tested using saliva for back-to-work, 50 people would be erroneously told they were no longer infectious.
What this means
In other studies, saliva collection has been reported to perform as well or better than NPS.1,2 Unfortunately, our results don’t support these findings. One possible explanation is the difference in the types of patients studied. Other studies have focused largely on inpatient populations, of which a large proportion were ICU patients. We focused primarily on patients in the community who were being tested in an outpatient setting – only two patients in the entire study were inpatients, and neither was admitted to the ICU. Outpatients are likely to have milder symptoms and have been shown to have reduced viral titers relative to more acute patients.5,6 Our findings are consistent with a large community study of 622 patients with paired NPS and saliva samples, where saliva samples were also found to be less sensitive in this setting.7 Before large-scale screening using saliva is initiated, we believe more rigorous studies in the intended population are needed.
What’s next
While we are disappointed by these results, we are committed to making the data available to the research and medical community to better inform important decision making – the pre-print, including detailed methods and supplemental data, is available on medRxiv and our team is happy to share more information. We are also continuing to do additional research to assess the viability of other sample types and testing approaches with the potential to enable massively scalable community-based testing.
For more information on these efforts, get in touch with us at covid@helix.com.
References
- ACCELERATED EMERGENCY USE AUTHORIZATION (EUA) SUMMARY SARS-CoV-2 ASSAY (Rutgers Clinical Genomics Laboratory). [cited 1 May 2020]. Available: https://www.fda.gov/media/136875/download
- Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv. 2020; 2020.04.16.20067835.
- Note: we attempted to estimate the relative performance of saliva to NPS with Bayesian Latent Class Models
- CDC Criteria for Return to Work for Healthcare Personnel with Suspected or Confirmed COVID-19 (Interim Guidance) Accessed 5/8/2020.
- Liu Y, Yan L-M, Wan L, Xiang T-X, Le A, Liu J-M, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020. doi:10.1016/S1473-3099(20)30232-2
- Van Vinh Chau N, Lam VT, Dung NT, Yen LM, Minh NNQ, Hung LM, et al. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. medRxiv. 2020; 2020.04.27.20082347.
- Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a non-invasive specimen for detection of SARS-CoV-2. J Clin Microbiol. 2020. doi:10.1128/JCM.00776-20