How the BRCA genes changed how we think about — and treat — cancer
By Misha DS Rashkin, MS, CGC, Senior Genetic Counselor
It’s difficult to overstate the importance that the BRCA genes — BRCA1 and BRCA2 — have played in the story of modern genetic testing. They were identified in the early ’90s by Mary-Claire King, PhD (a story told in the movie Decoding Annie Parker). Since then, thousands of other genes have been identified, the Supreme Court has gotten involved, presidents have discussed genetic testing in the State of the Union, Hollywood has used genetics in countless plots, Angelina Jolie has shared her experience with the BRCA1 gene in The New York Times, and most importantly, many lives have been saved. The BRCA genes may not be the lead actors in modern clinical genetics, but they’re certainly in the ensemble cast. And to understand why the BRCA genes affect a person’s risk of cancer, you need to know a little more about how cancer happens in the first place.
Cancer and the BRCA genes
Our body is made of billions of cells, and our cells are made up of proteins. These cells are constantly growing, replicating, and dying away. In this process of growth and replication, the DNA has to be replicated for every cell. Sometimes, a mistake happens that leads to a misspelling in the DNA. Many things can cause these misspellings, including the environment or just luck. Some misspellings are not important. The gene can still make proteins that work well, which make cells that also function well. But sometimes the misspelling leads to proteins that don’t function, like a typo in a cookbook that ruins the whole recipe (imagine how upset you’d be if you were looking forward to beef stew and got borscht!). When this happens in a gene that is involved in regulating growth or replication, a cell can start to replicate too much. This uncontrolled replication can then lead to a tumor.
The BRCA (“BReast CAncer”) genes play an important role in correcting these typos. They’re like editors that fix the misspellings in the DNA before they lead to tumors. But what if the editor has a typo in its DNA? It can’t do its job. In that case, the person’s odds of developing cancer go up, and sometimes it can happen earlier in life than it would in the general population. As the name implies, BRCA1 and BRCA2 play a particularly big role in the odds of developing cancer in the breast (in women and men; yes, men do have some small amount of breast tissue). It also affects cancer risk of the ovaries (in women), prostate (in men), and pancreas. Note that many genes have this characteristic where they affect more than one trait. Sometimes they are all related to one type of trait — like the odds of cancer in various organs — but sometimes they can affect traits that seem random and disconnected. In genetics, we call this “pleiotropy,” and it is the reason why the very name of the BRCA gene is inaccurate. When the genes were first discovered, the research was done in a population of women who had a significant personal and family history of breast cancer. Over time, we learned that variants in the BRCA genes affect more organs, so maybe they should be called the “BROVProPanCA” genes. Nonetheless, the “BRCA” name has stuck around.
And while we are busy introducing vocabulary terms, typos in genes are often referred to as “variants.” You can think of the DNA as a language that has four letters: A, T, C, G. If there’s a misspelling in the DNA, like an A being in the place of a T, or a couple letters being added or deleted, we call that a “variant.” Having a variant in BRCA1 or BRCA2 increases the likelihood of cells dividing and changing rapidly, and that in turn can increase the risk of certain types of cancers.
It does not, however, become a certainty that if someone has a variant in a BRCA gene that he or she will develop cancer; rather, the odds increase over the baseline for the general population. Below is a table that compares the general population’s odds of developing cancer in a specific organ against the odds if someone has a variant in BRCA1 or BRCA2. You’ll notice that there’s a big range for some of the percentages under BRCA1 and BRCA2. That’s because the research is still developing and different studies have reported different estimates. It may also be that the numbers are different for a particular person depending on their environment, family history, ethnicity, and possibly other genes that may modify the risks.
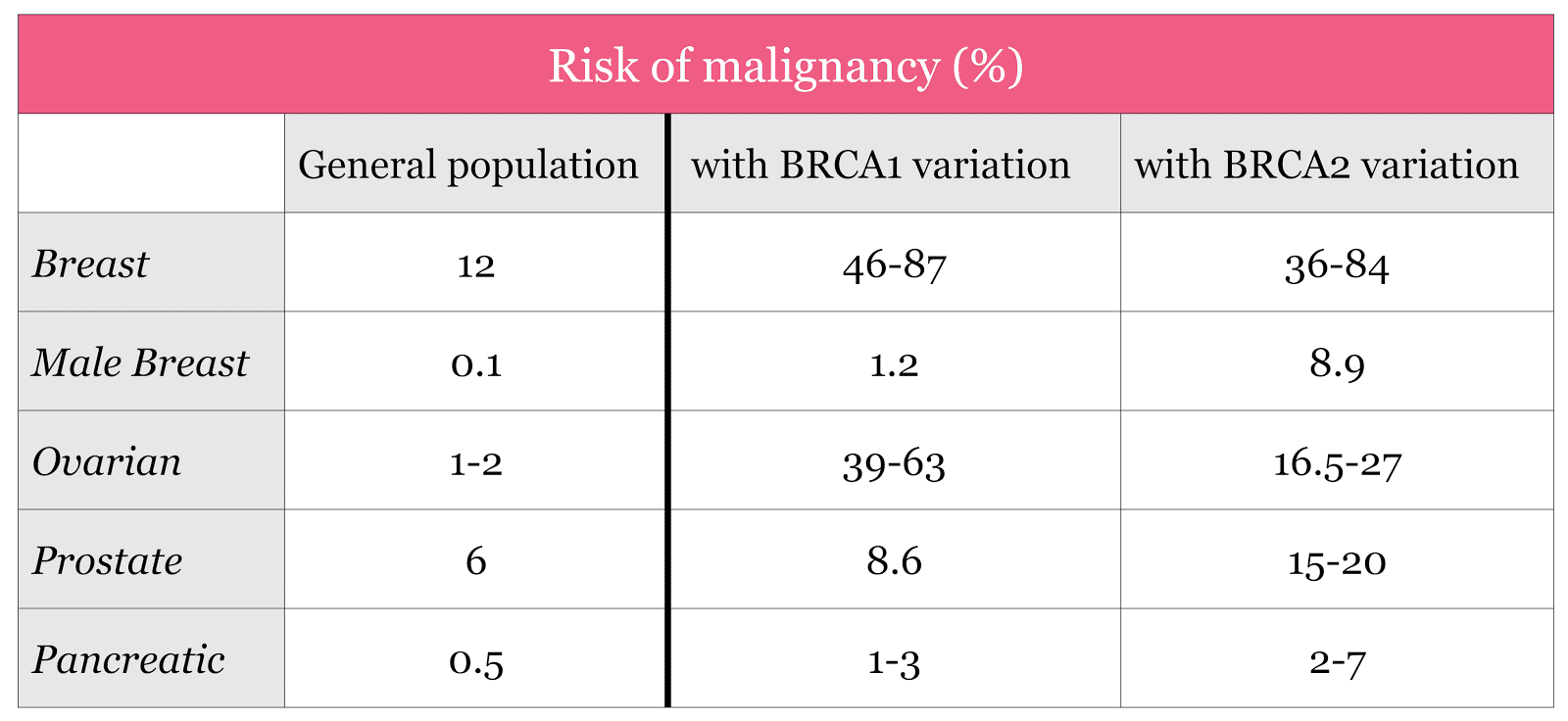
Source: Petrucelli et al. GeneReviews; accessed Aug 9th 2017
Now I know. What can I do?
So what can people do once they know they have a variant in BRCA1 or BRCA2? The options are different depending on the organ. We’ll focus on the breast, ovary, prostate, and pancreas. Men and women are advised to be sure they know how to do a thorough breast-self exam, do them monthly, and inform their doctors of their genetic test results. Women can also screen more regularly with mammograms and breast MRIs (some men may choose to do these as well). A woman could take a medication like tamoxifen, which affects how estrogen works in the body, and can lower the odds of breast cancer. Or they could choose to have a preventative mastectomy, in which breast tissue is surgically removed, with the option to have reconstructive surgery following the mastectomy.
For the ovaries, because a BRCA variant can increase the odds of cancer of the ovary or fallopian tube, some women will choose to have them removed, possibly after their childbearing years. This preventative approach has saved a lot of lives. In spite of the fact that the BRCA genes are often referred to as “the breast cancer genes,” the impact that the testing has had on saving the lives of women who could have developed ovarian cancer is possibly more significant. This is because ovarian cancer is often much harder to treat than breast cancer, and therefore more lethal. Interestingly, some research has suggested that having the ovaries removed before menopause can also decrease the risk for breast cancer for women who have a BRCA variant. On the flip side, keeping the ovaries can have benefits too. The ovaries are a primary source of estrogen, which is important for long-term health, including bone and heart health. So some research has begun to look into whether removing the fallopian tubes alone can prevent the onset of ovarian cancer. If this proves out, many women could get the benefits of ovarian cancer prevention without having to lose the ovaries as a primary source of estrogen.
For the prostate health, men can start having their prostate exams earlier than the general population, around 40 or 45 (men typically start at 50). Screening early is important because prostate cancer can have an earlier onset and can also be more aggressive among men when they have a BRCA2 variant. There are fewer screening and prevention options for pancreatic cancer at this time. Currently, participating in research for pancreatic cancer and avoiding smoking are the main things that people can do, although there is a lot of active research in the field (many studies are listed at www.clinicaltrials.gov).
Other genes
The BRCA genes are not the only genes that affect the risk of breast, ovarian, prostate, or pancreatic cancer, and there are genes that can affect the odds of developing cancer in other organs too. Researchers have identified dozens of other genes that can affect a person’s odds. They sort of read like an eye exam: CDH1, PTEN, P53, ATM, CHEK2 are just some examples.
Up until May of 2013 it wasn’t possible to put together a panel of genes including both BRCA1 and BRCA2, which was a problem if someone had a family history of breast cancer and wanted to be tested for all the genes at once. Why wasn’t it possible? Until that time, it was legal to patent a discovered gene, and the company that had the patent wasn’t sharing. You may be thinking, “but that’s crazy,” and luckily, the Supreme Court agreed. But until this decision, if any lab other than the one that held the patent wanted to make a panel targeting breast cancer, it couldn’t use the BRCA genes in its panel. That’s sort of like selling someone a car without a muffler, or an anthology of ’60s music without including the Beatles. Within a few months of the decision, multiple labs had panels and the whole industry was revolutionized.
Testing negative
Most people who have genetic testing for cancer predisposition test negative, and it’s often a relief. Other times, though, people have mixed feelings because they were hoping to solve a mystery, like why they developed cancer in the first place, or why many of their relatives have had it. These are normal emotions, and can sometimes be a sign that grief counseling is worth pursuing. It’s also important to understand that genetics is still a relatively new science, and new genes are still being discovered. So although testing negative lowers the odds that there’s something genetic or hereditary in play, it doesn’t eliminate the possibility altogether, because new genes are still yet to be discovered. So if you’ve had testing over the past few years, you might consider checking in with the doctor or genetic counselor who ordered your test to see if update testing is right for you.
Family
The BRCA genes, as well as most cancer predisposition genes, are inherited as “autosomal dominants.” “Autosomal” refers to genes that can come from mom or dad and be passed onto sons or daughters. “Dominant” means that it only takes one copy of the gene having a variant for a person to develop the disease or have the predisposition. Since parents can only pass on half their genetic material to their kids, it’s a 50/50 chance that a man or woman who has a variant in a cancer predisposition gene will pass it on to their child. And the gender of the child also doesn’t matter, although obviously the impact can be different. So if a father passes on a gene that increases the odds of ovarian cancer to a boy, or prostate cancer predisposition to a girl, that’s not really a problem since boys don’t have ovaries and girls don’t have prostates.
Once someone tests positive for a variant, it’s important that they tell their relatives because then the relative has the opportunity to get tested and see if they inherited the same variant, which could help them screen, prevent, or possibly even treat (more on treatment in a minute) a future cancer. And when a person tests negative, it’s still possible that other relatives could test positive because of the 50/50 odds of passing a gene with a variant. For this reason, it’s often the most efficient to test the person who had the cancer diagnosis, if they are interested and available.
Treatment
BRCA testing has played a huge role in prevention, to the point where people who have tested positive for a variant and taken preventive action have created a new word for themselves — previvors (like “survivor” but “pre”). But now BRCA testing is starting to play a part in treating cancer too. In the past few years, some studies have shown that women who have ovarian cancer and a BRCA variant can benefit from a specific type of chemotherapy called a PARP-inhibitor. It may turn out that PARP-inhibitors can be effective for people with BRCA variants in other cancer types as well, such as pancreatic cancer which is currently very hard to treat. Additionally, some people are now being tested for genetic variants shortly after being diagnosed with cancer to help them decide on which type of treatment to pursue.
The future
As you can see, there’s a lot to the story here! Genetic testing for cancer susceptibility has created tremendous opportunities for prevention, but it’s also put people in the position of making difficult decisions. An entire new industry has developed consisting of specialized cancer genetic counselors who can help you discuss this topic in detail, and many doctors have made it a specialty to advise patients as well. Some hospitals have even started specialized clinics, and new support and advocacy groups are available. It’s definitely complicated, but if you’re thinking about testing, or making decisions based on testing results, you’re not alone and there are many resources for you.
For more information on BRCA, visit the National Cancer Institute’s Fact Sheet on BRCA1 and BRCA2: Cancer Risk and Genetic Testing. For more information on Helix, visit helix.com.